What Does It Mean When We Say An Atom Has Decayed. Since the water molecule comprising two atoms of Hydrogen and one of Oxygen is formed by covalent bonds the electrons are shared. And yes the wave function collapses only when the observer gets a definitive report as to whether the atom has decayed.
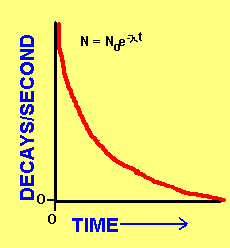
A substituent with a higher atomic number takes precedence over a substituent with a lower atomic number. This means that the atom releases energy either in alpha beta or gamma form. The net charge on either metal will be different due to different reaction rates so charge will flow from one to the other if there is a path.
By showing the percentage of each type of carbon an artifact has.
For example Uranium-235 decays when struck by a neutron and will usually turn into 3 neutrons and nuclei like barium krypton and xenon isotopes. No you lose about half each time. When an atom decays it shall usually emit some form of radiation eg. An excited atom is one that does not have its electrons in the ground state.
