Xeo4 Lewis Structure. I know there are a totalof 32 valence electrons and that Xe forms double bonds with O butI dont understand why and an explaination would be great. Xenon having valence electrons in the 4th energy level will also have access to the 4d sublevel thus allowing for more than 8 electrons.
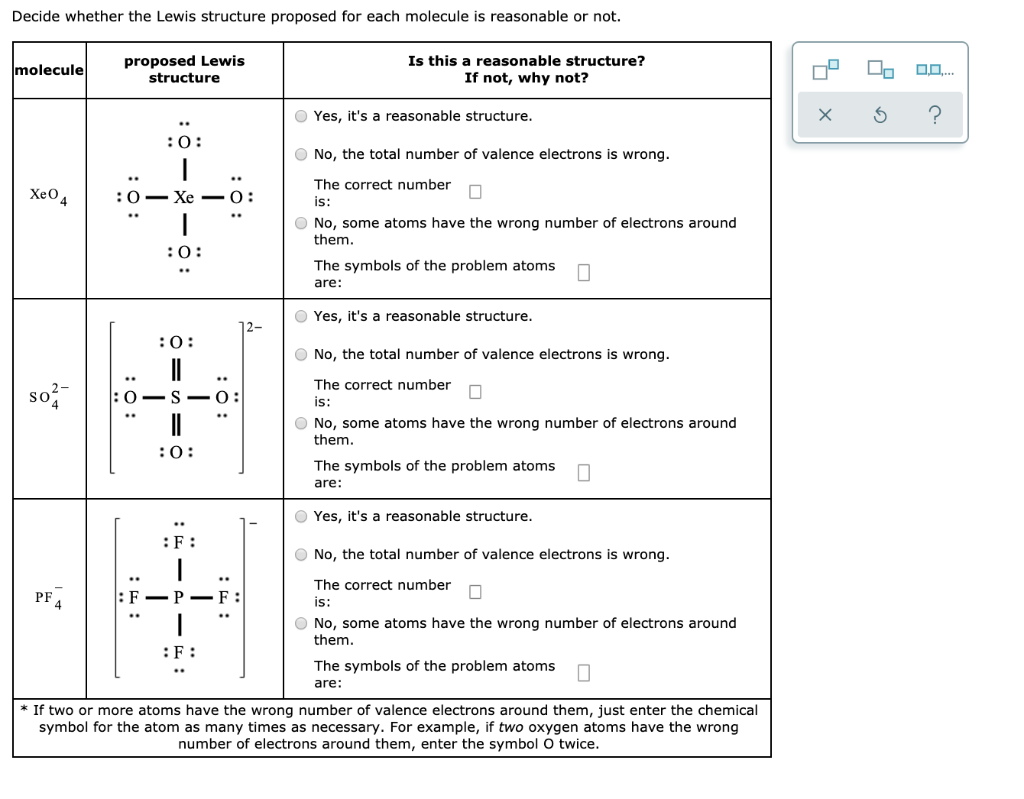
The lone pairs in the molecule are located in a perpendicular plane in an octahedral shape to keep their repulsive forces at a minimum. Since it has no lone pair of electrons the shape of XeO4 is tetrahedral with the bond angle of 109 degrees. Id be glad if you could help me with this.
Molecular Geometry Of Xeo4.
On each side draw a single bond. A Determine the total number of valence electrons. Itd even better if you actually gave a recap of how-to-draw-noble-gas-compounds as a Lewis Structure. To find the number of valence electron you get in one molecule of xenon oxytetrafluoride add the number of valence electrons of each individual atom that makes up the molecule.
